Cathodic Protection: it 2 Types, Applications & How It Works?
Cathodic protection is used to protect numerous structures against corrosion like ships, offshore floaters, subsea equipment, harbors, pipelines, and tanks, etc.
To mitigate corrosion damage to active metal surfaces, cathodic-protection is often used and it also protects reinforcement bars in concrete structures and piers.

When two dissimilar metals are submerged in an electrolytic substance such as water, soil, or concrete corrosion is caused and metals act as the anode without cathodic protection and easily lose their electrons. So, cathodic-protection simply supplies the metal with electrons from an external source and making it a cathode.
How does Cathodic Protection work?
By placing an anode or anodes (external devices) in an electrolyte to create a circuit cathodic protection works and to the surface of the structure, current flows from the anode through the electrolyte.
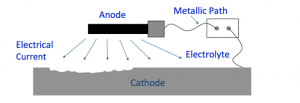
To stop further corrosion of the structure, corrosion moves to the anode.
Types of Cathodic Protection:
There are two main types of cathodic-protection as given below;
- Galvanic Cathodic Protection
- Impressed Current Cathodic Protection (ICCP)
Galvanic Cathodic Protection:
Galvanic cathodic protection is a protection of the metal surface of a piece of equipment using another metal that is more reactive. The latter metal usually called the galvanic or sacrificial anode which has a less negative electrochemical potential.
Galvanic anodes have more negative electrode potential than the metal of the target structure and also have limited life-spans during which the sacrificial anode will continue to degrade and protect the piping or the tank. Until the anode material is fully consumed, the galvanic anode keeps corroding.
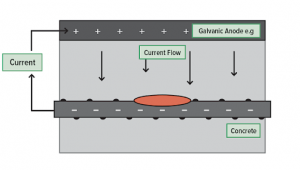
Sometime galvanized steels are used and these steels are coated with a protective zinc layer and in most underground and marine environments, the zinc layer acts to cathodically protect steel against corrosion.
Impressed Current Cathodic Protection: (ICCP)
Impressed current cathodic protection is a more economical method of cathodic protection when underground pipelines are offshore or long equipment is too large to protect via one or few galvanic anodes and electrons are supplied to the cathodic structure using an external DC power source.
This type has a much longer life span than the galvanic anodes and includes a rectifier that converts the AC power source to a DC. With the impressed current system, an anode is also required but it can be of an inexpensive material like scrap steel or graphite and in an impressed current system, there is practically no limit on the current output.

To the negative terminal of the power source, the steel component is connected and to the positive terminal of the power source, the impressed current anodes are connected.
Applications of Cathodic Protection:
There are the following applications as given below;
Submarine Pipelines:
By either aluminum or zinc anodes in bracelet and slander form these pipelines are usually protected and installed at regular intervals.

The pipelines may be protected by an Impressed Current System in some cases and the system can be operated either independently or tied with other structures such as a platform. The choice of the anode type would be determined by the environmental conditions, maintenance, and installation constraints in these cases.
Hot Water Tank / Water Heater:
To protect water heaters, this technology is used and the electrons sent by the imposed current anode prevent the tank from rusting inside it.

Marine:
This covers many areas such as jetties, harbors, offshore structures, and to provide protection, a variety of different types of structure leads to a variety of systems.
Ship Hulls:
This protection is normally applied to the whole underwater hull which includes the typical cavitation and corrosion-prone areas around the stern.

Like seawater inlet boxes where shielding from exterior hull protection may occur anodes are also fitted.
Steel in concrete:
At the time of construction when the concrete is being poured, the anodes and reference electrodes are usually embedded in the concrete, and ICCP is commonly used as a usual technique for concrete buildings, bridges, and similar structures.

